Exploring a parasitic tunnel boring machine
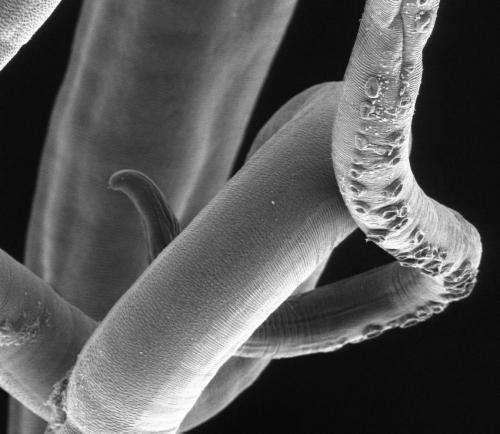
Researchers have deduced essential biological and genetic information from the genome sequence of the whipworm, an intestinal parasitic worm that infects hundreds of millions of people in developing countries.
This information acts as the foundation for the development of new strategies and treatments against this debilitating parasite.
The whipworm is one of three types of soil-transmittedparasitic wormsthat collectively infect nearly two billion people. While infections often result in mild disease they may also lead to serious and long-term damage such as malnutrition, stunted growth and impaired learning ability. The full extent of worm-associated morbidity and the effect it has on socio-economic development in endemic countries is unknown.
This unusual parasite bores miniature tunnels through the lining of the large intestine where it may live for years. The study has identified molecules that the parasite uses for tunnelling, how the parasite limits the damage it inflicts, and how the immune system responds to infection.
"Worm infections are an enormous public health problem across the Developing World and with so few effective drugs, the emergence of drug resistance is an ever present risk," says Dr Matthew Berriman, senior author from the Wellcome Trust Sanger Institute. "Our work starts to unravel the whipworm's intimate relationship with humans and paves the way for new approaches to prevent or clear whipworm infections.
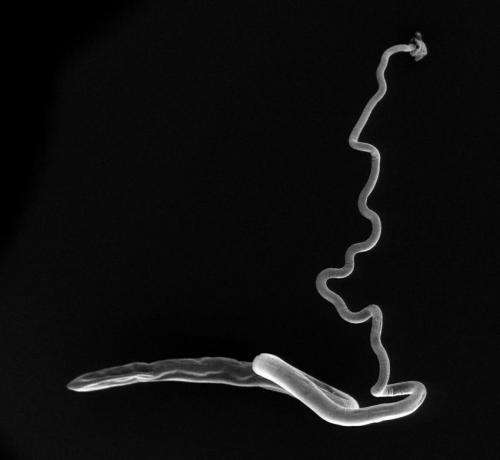
"Making these genome sequences freely available will provide an enormous boost to the entire research community that is working on interventions to prevent or treat worm-associated disease."
The team sequenced the genome of both a human- and a mouse-infective form of the whipworm and examined the genes that are most active and may be essential for its survival. Equipped with this information, the team mined for drug targets that could be used against the whipworm and potentially other parasitic worms.
Thegenome sequenceand the range of proteins the whipworm produces provides a biological understanding of the extraordinary niche this worm has evolved to live in. The team found specific digestive enzymes secreted by the whipworm may burrow through the cells in the gut wall. Other enzymes secreted by the parasite seem to contain the 'collateral damage' caused by thesedigestive enzymesto reduce inflammation and damage to the host's cells.
"In my experience working with children in Ecuador, these parasites, particularly when present in large numbers in an individual, can have profound effects on health," says Professor Philip Cooper, author and clinician from the Liverpool School of Tropical Medicine. "With more than 800 million children worldwide in need of treatment against these particular worms, and because we have only one or two drugs that are safe and effective against these parasites, it is essential that we focus our research on finding new treatments before resistance to the drugs we have has a chance to develop. This study not only opens doors for the development of new drugs but also may allow us to identify already existing drugs used for other diseases that might be effective against this parasite and other types of worms.
"Getting to grips with the genomes and the underlying biology of parasitic worms such as the whipworm is our best option to tackle this growing global problem."
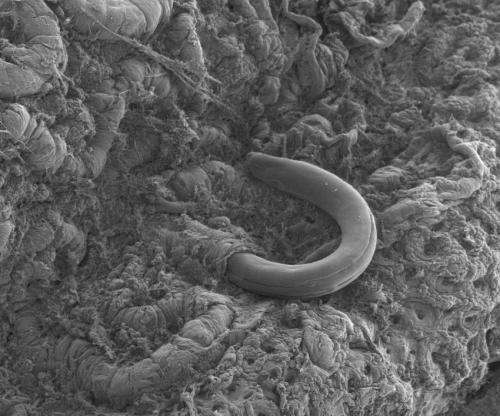
Whipworm eggs are currently being tested in clinical trials as a treatment against various autoimmune diseases, as it is thought that worm infections may reduce the inflammation associated with disorders such as multiple sclerosis and inflammatory bowel disease. To determine how the immune system responds to infection, mice were exposed to the mouse-specific type of whipworm. The researchers found that infection caused changes to the activity of many mouse genes that have been associated with inflammatory diseases such as ulcerative colitis.
"Although whipworms can be detrimental to human health and economic growth in some regions, they are also important in defining our immune system's 'set point' and ensuring we make the right level of immune response during disease," says Professor Richard Grencis, senior author from The University of Manchester. "The present study shows how both the parasite and the host respond to each other at a level of detail never seen before, that will help us identify how to exploit the ways in which the worm modifies our bodies.
"This finely tuned interaction that has developed over the course of evolution can lead us to design better drug treatments and more effective clinical trials using worms and their products."
Explore further