Protein that repels immune cells protects transplanted pancreatic islets from rejection
An approach developed by Massachusetts General Hospital (MGH) investigators may provide a solution to the limitations that have kept pancreatic islet transplantation from meeting its promise as a cure for type 1 diabetes. In the March issue of theAmerican Journal of Transplantation, the research team reports that encapsulating insulin-producing islets in gel capsules infused with a protein that repels key immune cells protected islets from attack by the recipient's immune system without the need for immunosuppressive drugs, restoring long-term blood sugar control in mouse models. The technique was effective both for islets from unrelated mice and for islets harvested from pigs.
"Protecting donor islets from the recipient's immune system is the next big hurdle toward makingislet transplantation一个真正的治愈1型糖尿病,”马克·波兹南说sky, MD, PhD, director of the MGH Vaccine and Immunotherapy Center, who led the study. "The first was generating enough insulin-producing islets, which has been addressed by several groups usingpig isletsor - as announced last fall by Doug Melton's team at the Harvard Stem Cell Institute - with isletcellsderived from human stem cells. Now our technology provides a way to protect islets or other stem-cell-derived insulin-producing cells from being destroyed as soon as they are implanted into a diabetic individual without the need for high-intensity immunosuppression, which has its own serious side effects."
While transplantation ofpancreatic isletshas been investigated for several decades as a treatment and potential cure fortype 1 diabetes, its success has been limited. Along with the risk of rejection that accompanies all organ transplants - a risk that is even greater for cross-species transplants - donated islets are subject to the same autoimmune damage that produced diabetes in the first place. The immunosuppressive drugs used to prevent organ rejection significantly increase the risk of infections and some cancers, and they also can contribute directly to damaging the islets. Among the strategies investigated to protect transplanted islets are enclosing them in gel capsules and manipulating the immune environment around the implant. The MGH-developed approach includes aspects of both approaches.
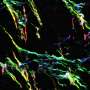
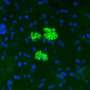
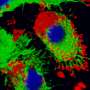
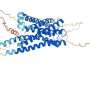
Previous research from the MGH team demonstrated that elevated expression of a chemokine - a protein that induces the movement of other cells - called CXCL12 repels the effector T cells responsible for the rejection of foreign tissue while attracting and retaining regulatory T cells that suppress the immune response. For the current study they investigated how either coating islets with CXCL12 or enclosing them in CXCL12 gel capsules would protect islets transplanted into several different mouse models.
Their experiments revealed that islets from nondiabetic mice, either coated with CXCL12 or encapsulated in a CXCL12-containing gel, survived and restored long-termblood sugarcontrol after transplantation into mice with diabetes that was either genetically determined or experimentally induced. CXCL12-encapsulated islets were even protected against rejection by recipient animals previously exposed to tissue genetically identical to that of the donor, which usually would sensitize theimmune systemagainst donor tissue. CXCL12-encapsulated pig islets successfully restoredblood sugar controlin diabetic mice without being rejected. The ability of CXCL12 - either as a coating or encapsulating gel - to repel effector T cells and attract regulatory T cells was also confirmed.
"While studying this procedure in larger animals is an essential next step, which is currently underway with the support of the Juvenile Diabetes Research Foundation, we expect that this relatively simple procedure could be readily translatable into clinical practice when combined with technologies such as stem-cell-derivedisletsor other insulin-producing cells and advanced encapsulation devices," says Poznansky, an associate professor of Medicine at Harvard Medical School. "We also hope that CXCL12 will have a role in protecting other transplanted organs, tissues and cells as well as implantable devices, a possibility we are actively investigating."