New insights into brain circuit for hunger responses during starvation
Researchers uncover mechanism by which hypothalamic neural signaling drives hunger responses to survive starvation.
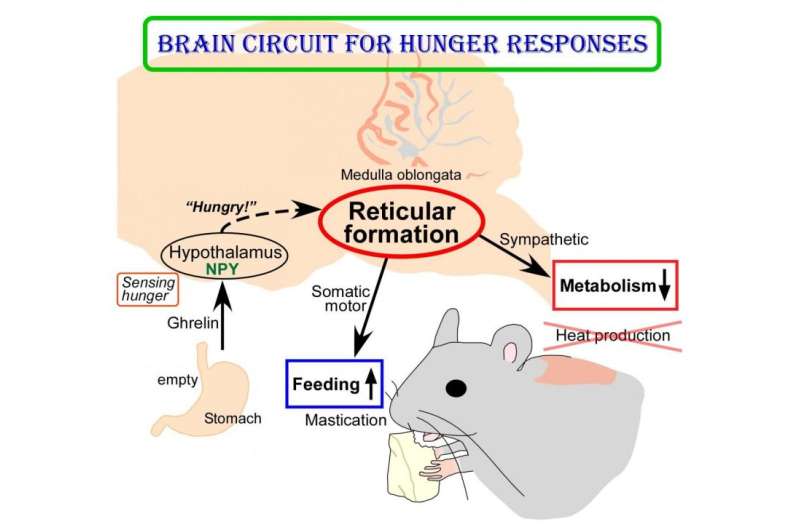
The human body responds to starving conditions, such as famine, to promote the chance of survival. It reduces energy expenditure by stoppingheat productionand promotes feeding behavior. These "hunger responses" are activated by the feeling of hunger in the stomach and are controlled by neuropeptide Y (NPY) signals released by neurons in the hypothalamus.
However, how NPY signaling in the hypothalamus elicits the hunger responses has remained unknown.
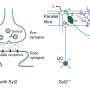
Sympathetic motor neurons in the medulla oblongata are responsible for heat production by brown adipose tissue (BAT). Researchers centered at Nagoya University have now tested whether the heat-producing neurons respond to the same hypothalamic NPY signals that control hunger responses. They injected NPY into the hypothalamus of rats and tested the effect on heat production. Under normal conditions, blocking inhibitory GABAergic receptors or stimulating excitatory glutamatergic receptors in the sympathetic motor neurons induced heat production in BAT. After NPY injection, stimulating glutamatergic receptors did not produce heat, but inhibiting GABAergic receptors did. The study was recently reported inCell Metabolism.
"This indicated that hypothalamic NPY signals prevent BAT thermogenesis by using inhibitory GABAergic inputs to sympathetic motor neurons," study lead author Yoshiko Nakamura says.
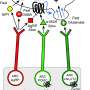
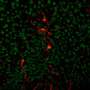
Retrograde and anterograde tracing with fluorescent dyes revealed which brain region provided the inhibitory GABAergic inputs to heat-producing motor neurons.
"Tracing experiments showed that sympatheticmotor neuronsare directly innervated by GABAergic inputs from reticular nuclei in the medulla oblongata," corresponding author Kazuhiro Nakamura explains, "selective activation of these GABAergic reticular neurons inhibits BAT thermogenesis."
The researchers' further findings showed that GABAergic inputs from medullary reticular neurons are involved in hypothalamic NPY-mediated inhibition of heat production in BAT. This hunger response circuit probably explains why anorexic individuals suffer from hypothermia.
Interestingly, stimulation of these medullary reticular neurons prompted rats to begin chewing and feeding. This effect was similar to injecting NPY into the hypothalamus, suggesting that hypothalamic NPY signaling activates reticular neurons in the medulla oblongata to promote feeding and mastication during the hunger response.
Abnormal activation of theseneuronsunder non-starved conditions may contribute to obesity. Understanding these mechanisms could lead to development of more effective treatments for obesity.
The novel brain circuit for hunger responses is illustrated in the accompanying figure.
Explore further