Assay can ID M. tuberculosis resistance mutations
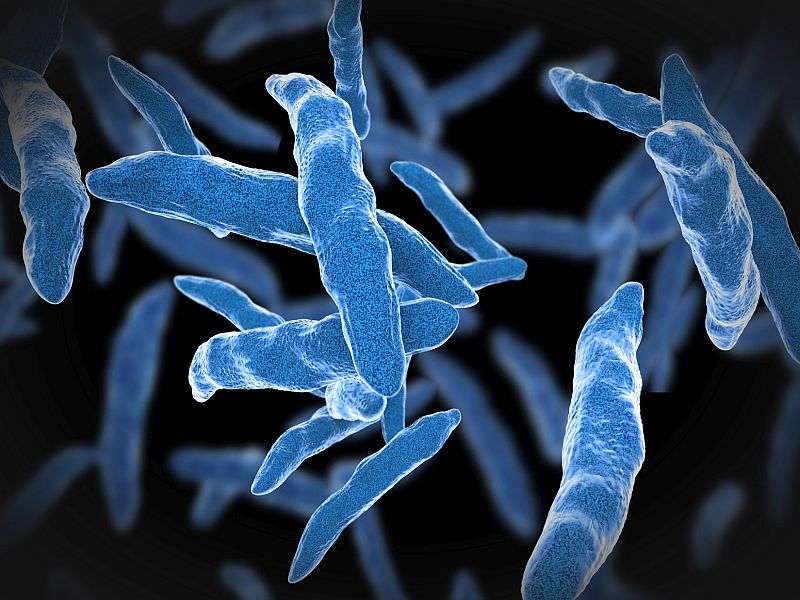
(HealthDay)—An automated molecular assay can detectMycobacterium tuberculosis直接从痰规范与抗药性imens, according to a study published in the Sept. 14 issue of theNew England Journal of Medicine.
Yingda L. Xie, M.D., from the National Institutes of Health in Bethesda, Md., and colleagues compared the investigational assay against phenotypic drug-susceptibility testing and DNA sequencing among adults with symptoms of tuberculosis. The authors performed the Xpert MTB/RIF assay and sputum culture.
The researchers found that the sensitivities of the investigational assay for detecting resistance were 83.3 percent for isoniazid, 88.4 percent for ofloxacin, 87.6 percent for moxifloxacin, 71.4 percent for kanamycin, and 70.7 percent for amikacin among the 308 participants who were culture-positive forM. tuberculosiswhen phenotypic drug-susceptibility testing was used as the reference standard. For detection of phenotypic resistance the specificity of the assay was 93.4 percent or greater for all drugs except moxifloxacin at a critical concentration of 2.0 µg/mL. Using DNA sequencing as the reference standard, the sensitivities of the investigational assay for detecting mutations associated with resistance were 98.1, 95.8, 92.7, and 96.8 for isoniazid, fluoroquinolones, kanamycin, and amikacin; the specificity for all drugs was 99.6 percent or greater.
"This investigational assay accurately detectedM. tuberculosismutations associated withresistanceto isoniazid, fluoroquinolones, and aminoglycosides and holds promise as a rapid point-of-care test to guide therapeutic decisions for patients with tuberculosis," the authors write.
Explore further
Copyright © 2017HealthDay. All rights reserved.










User comments