Cancer research reveals how mutations in a specific gene cause different types of disease

Leading cancer experts at the University of Birmingham have solved a long-standing question of how various types of mutations in just one gene cause different types of diseases.
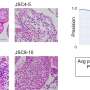
A team of scientists at the University's Institute of Cancer and Genomic Sciences, led by Professor Constanze Bonifer, studied a gene known as RUNX1, which is responsible for providing instructions for the development of all血cellsand is frequently mutated in血cancers.
The results of their research has shown that the balance of cells types in the blood is affected much earlier than previously thought, which is particularly important for families that carry the mutant gene.
The research, published inLife Science Alliance, opens up the possibility of identifying early changes in cells of patients carrying the mutation even before anydiseasemanifests itself—increasing their chances of survival.
The study, the culmination of four years of research, showed that whilst some types of RUNX1mutationsdirectly changed how othergenesbehaved in blood cells, not all did. In particular, the mutations that are inherited through families do not immediately affect the cells but instead change the roadmap they follow to become other cell types, such as platelets and white blood cells.
Lead author Professor Constanze Bonifer said: "The most important results we found came from studying mutations that run in families which predisposes their members to diseases such as Familial Platelet Disorder (FPD) and Acute Myeloid Leukemia (AML).
"AML is an aggressivecancerof the white blood cells, whereas in FPD, the ability to produce blood clots which is required to stop bleeding is impaired. Prior to this study, it was completely unclear why changes in just one gene cause so many different diseases."
Co-corresponding author Dr. Sophie Kellaway said: "We used a cell culture system capable of generating blood cells in vitro, then induced the mutant forms of RUNX1 in these cells and immediately examined the effect on cellular behavior and gene activity.
"We found that every RUNX1 mutation changed cells in a different way and had a different impact on how genes responded.
"What we have been able to demonstrate is that different genetic alterations in RUNX1 can send cells towards alternate paths of malignancy."
This work was funded by grants from the Kay Kendall Leukemia Fund, the Biotechnology and Biological Sciences Research Council, and Blood Cancer UK.
Rachel Kahn, Research Communications Manager at Blood Cancer UK said: "This detailed research shows that it's not only a mutation that's important in deciphering whether or not someone will develop a disease, but it's precisely where the mutation occurs that can alter how blood cells develop and lead to disease.
"Many血cancersare difficult to treat and have a poor prognosis. This is particularly the case for AML, which was studied in this research. Understanding more about what specific changes lead to the disease will help us to tailor treatments in the future, giving everyone the best possible chance of survival."
The research results demonstrate that different classes of mutant RUNX1 proteins use unique multifactorial mechanisms to cause disease and so development of novel treatments will require an individual approach.
The team now plans to work with clinicians and families carrying mutant RUNX1 proteins, to examine patient blood cells to see whether their findings in cultured cells can also be seen in patient bloodcells, in particular, before they develop any symptoms. They will then examine whether they can find ways to restore normal blood cell development.







User comments