Registry data reveal safety of COVID-19 vaccines in people with rheumatic and musculoskeletal diseases
In general, people with inflammatory or autoimmune rheumatic and musculoskeletal diseases (RMDs) have not been included in the clinical trial programs for COVID vaccines. This has led to questions about the safety and effectiveness of COVID vaccines in people with inflammatory RMDs. The COVAX registry aims to answer these questions and provide information about the safety of COVID vaccination in people with RMDs. This latest publication reveals data from over 5,000 people across a number of different RMDs and vaccine types. The findings should provide reassurance to health professionals and vaccine recipients, and promote confidence in the safety of COVID vaccination in people with inflammatory RMDs.
Since SARS-CoV-2 emerged at the end of 2019, the virus has caused a global pandemic. In February 2021, EULAR, the European Alliance of Associations for Rheumatology, launched COVAX—a physician-reported registry to collect information about COVID vaccination inpeoplewith both inflammatory and non-inflammatory RMDs. Rheumatologists in EULAR-affiliated countries were asked to report as many cases as possible of people with RMDs who had received a COVIDvaccine, whether or not they had experienced side effects.
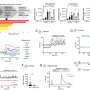
From February to July 2021, information was collected for 5,121 people with different types of RMD who had received at least one dose of a COVID vaccine. The most common inflammatory RMDs wererheumatoid arthritis, axial spondyloarthritis, and psoriatic arthritis. The most common non-inflammatory RMDs were osteoarthritis and osteoporosis. Among people with inflammatory RMDs, 54 percent were taking a conventional synthetic disease-modifying antirheumatic drug (csDMARD), 42 percent were on biological DMARDs (bDMARDs), and 35 percent were taking immunosuppressant medicines for their RMD (e.g., glucocorticoids, mycophenolate, azathioprine).
The results from COVAX show that the majority of people with inflammatory RMDs tolerate their COVID vaccine well—with no difference in safety profile to that seen in thegeneral populationor people with non-inflammatory RMDs. The most common side effects were short-lived reactions to the injection. One of the reasons for asking additional safety questions in people with inflammatory RMDs is the concern that the vaccine could cause a disease flare. In this study, only 4.4 percent of people experienced a flare after having their COVID vaccine, and only 0.6 percent were classed as severe. The majority of people (over 98 percent) were able to continue on their normal medication with no changes. The study also found there was a low rate of COVID-19 infections in people with RMDs once they were fully vaccinated.
These are valuable findings which will support discussions about the safety and benefit/risk ratio of COVID vaccination for people with RMDs. The information will also be useful for the development of new and updated recommendations.
More information:Pedro M Machado et al, Safety of vaccination against SARS-CoV-2 in people with rheumatic and musculoskeletal diseases: results from the EULAR Coronavirus Vaccine (COVAX) physician-reported registry,Annals of the Rheumatic Diseases(2021).DOI: 10.1136/annrheumdis-2021-221490