T-cell interactions vary between tumor microenvironments
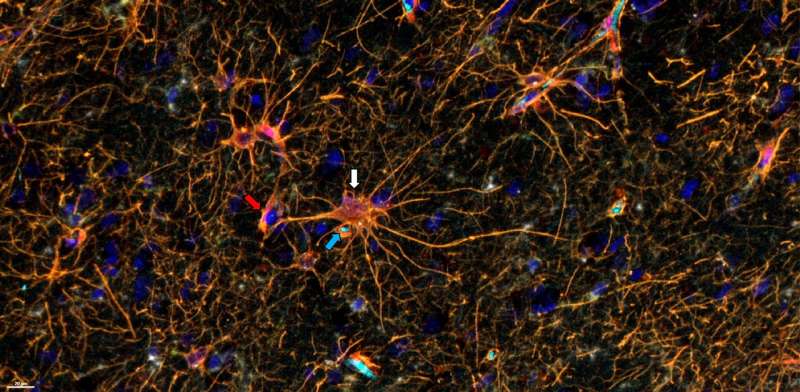
A team led by Northwestern Medicine investigators has discovered differences in the distribution and interaction of T-cells within the microenvironment of different regions of both brain tumors and brain metastases, according to findings published in the journalJCI Insight. The findings demonstrate how the immune cell interactome is distinct between cancer lineages.
"These interactions likely influence how we generate immune responses to cancer intrinsically," said Amy Heimberger, MD, the Jean Malnati Miller Professor of Brain Tumor Research and senior author of the study.
The body's immune system responds to brain tumors through the lymphatic drainage of tumor antigens, in whichdendritic cells, or antigen-presentingimmune cells, present tumor antigens in thelymph nodesto T-cells, which are then activated. These T-cells then traffic to thetumor microenvironmentwhere they mediate tumor cell killing.
Previous studies have shown that trafficking to the tumor microenvironment exhausts T-cells in cancers that reside in the brain, causing them to become less effective or even inactive once they reach the tumor. According to Heimberger, some of these cluster interactions may indicate a benefit to initiating T-cell activation prior to T-cell exhaustion.
In the current study, Heimberger's team aimed to determine the degree to which T-cells are distributed and interact within different regions of brain tumors, including regions of cell death or necrosis and at the infiltrating edge, or where healthy brain tissue and the tumor meet.
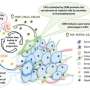
Compared to recent studies which have explored the tumor microenvironment and its immune composition through tumor biopsies, Heimberger's team utilized en bloc resections of glioma tumors andbrain metastasesfrom lung cancer. Unlike a traditional biopsy, these resections preserve tissue structure, orientation and architecture throughout all areas of the tumor's microenvironment.
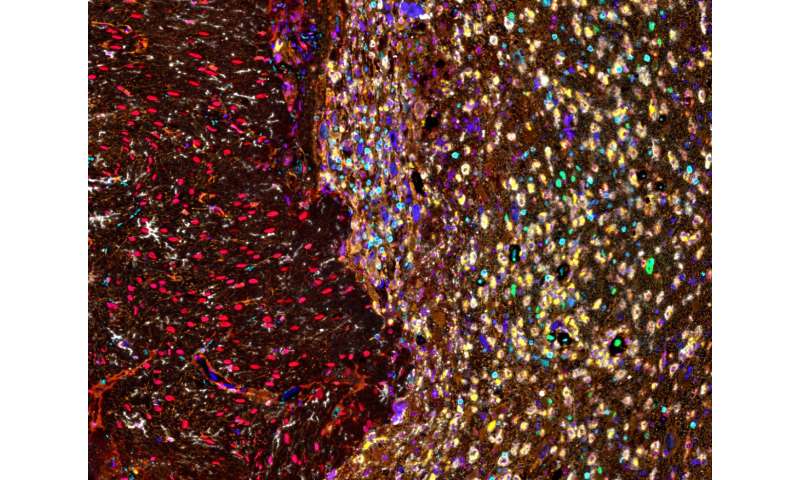
"We analyzed these cellular interactions in linearity and continuity within the tumor microenvironment. The tumor microenvironment is not only tumor, but also the tumor edge and necrosis," said Hinda Najem, MD, MS, a postdoctoral fellow in the Heimberger laboratory and lead author of the study. "Because we examined the tissue in continuity, we were able to see that tumors in the brain create a gradient of macrophages in the adjacent brain."
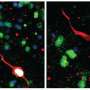
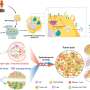
Using multiplex immunofluorescence staining and single-cell RNA sequencing, the team examined T-cell distribution and interactions within the primary and metastatic brain tumors, as well as the inferred functionality of these T-cells in the tumor microenvironment.
Overall, the investigators found stark differences in the tumor microenvironments, cancer lineage and interacting cellular partners with the T-cells. For example, within gliomas, T-cells were localized to the infiltrating edge and perivascular space of the tumors, but in metastatic tumors, T-cells were found in the stroma, which holds the tumor tissues together. They also found that immunosuppressive cells that express the transcription factor STAT3 tend to congregate together.
"This research highlights the point that when tumors metastasize, they seek locations enriched in the negative influences of the CD163+macrophages. We need to find ways to reorganize the cells to sequester these immunosuppressive cells from our tumor microenvironments or we need to reprogram them to be less immunosuppressive," said Jared Burks, Ph.D., co-director of the Flow Cytometry & Cell Imaging Core Facility at The University of Texas MD Anderson Cancer Center and a co-author of the study.
The study also lays the groundwork for evaluating the role of newly defined immune cell populations in braintumorgrowth and therapeutic responses.
"Therapeutics right now are very focused on targeting single cells. A future area of therapeutics could be considered that specifically modulate the relationships of cells," Heimberger said.
Explore further