Fighting viruses is as easy as breathing
The average person will take more than 600 million breaths over the course of their life. Every breath stretches the lungs' tissues with each inhale and relaxes them with each exhale. The mere motions of breathing are known to influence vital functions of the lungs, including their development in babies, the production of air-exchange-enhancing fluid on their inner surfaces, and maintenance of healthy tissue structure. Now, new research from the Wyss Institute at Harvard University has revealed that this constant pattern of stretching and relaxing does even more—it generates immune responses against invading viruses.
Using a Human Lung Chip that replicates the structures and functions of the lung air sac, or "alveolus," the research team discovered that applying mechanical forces that mimic breathing motions suppresses influenza virus replication by activating protective innate immune responses. They also identified several drugs that reduced the production of inflammatory cytokines in infected Alveolus Chips, which could be useful in treating excessive inflammation in the lung. Based on these studies, one of those drugs was licensed to Cantex Pharmaceuticals for the treatment of COVID-19 and other inflammatory lung diseases. Data from the research were recently included in the company's Investigational New Drug (IND) application to the FDA to initiate a Phase 2 clinical trial for COVID-19.
"This research demonstrates the importance of breathing motions for human lung function, including immune responses to infection, and shows that our Human Alveolus Chip can be used to model these responses in the deep portions of the lung, where infections are often more severe and lead to hospitalization and death," said co-first author Haiqing Bai, Ph.D., a Wyss Technology Development Fellow at the Institute. "This model can also be used for preclinical drug testing to ensure that candidate drugs actually reduce infection and inflammation in functional human lung tissue." The results are published today inNature Communications.
Creating a flu-on-a-chip
As the early phases of the COVID-19 pandemic made painfully clear, the lung is a vulnerable organ where inflammation in response to infection can generate a "cytokine storm" that can have deadly consequences. However, the lungs are also very complex, and it is difficult to replicate their unique features in the lab. This complexity has hindered science's understanding of how the lungs function at the cell and tissue levels, in both healthy and diseased states.
The Wyss Institute's Human Organ Chips were developed to address this problem, and have been shown to faithfully replicate the functions of many different human organs in the lab, including the lung. As part of projects funded by the NIH andDARPAsince 2017, Wyss researchers have been working on replicating various diseases in Lung Airway and Alveolus Chips to study how lung tissues react to respiratory viruses that have pandemic potential and test potential treatments.
During his Ph.D. training, Bai studied diseases that affect the tiny air sacs deep inside the lungs where oxygen is rapidly exchanged for carbon dioxide. That foundation prepared him to tackle the challenge of recreating a flu infection in an Alveolus Chip so that the team could study how these deep lung spaces mount immune responses against viral invaders.
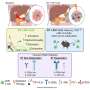
Bai and his team first lined the two parallel microfluidic channels of an Organ Chip with different types of livinghuman cells—alveolar lung cells in the upper channel and lung blood vessel cells in the lower channel—to recreate the interface between human air sacs and their blood-transporting capillaries. To mimic the conditions that alveoli experience in the human lung, the channel lined by alveolar cells was filled with air while the blood vessel channel was perfused with a flowing culture medium containing nutrients that are normally deliveredviathe blood. The channels were separated by a porous membrane that allowed molecules to flow between them.
Previous studiesat the Wyss Institute have established that applying cyclical stretching to Alveolus Chips to imitate breathing motions produces biological responses that mimic those observedin vivo. This is accomplished by applying suction to hollow side chambers adjacent to the cell-lined fluidic channels to rhythmically stretch and relax the lung tissues by 5%, which is what human lungs typically experience with every breath.
When the team infected these "breathing" Alveolus Chips with H3N2 influenza by introducing the virus into the air channel, they observed the development of several known hallmarks of influenza infection, including the breakdown of junctions between cells, a 25% increase in cell death, and the initiation of cellular repair programs. Infection also led to much higher levels of multiple inflammatory cytokines in the blood vessel channel including type III interferon (IFN-III), a natural defense against viral infection that is also activated inin vivoflu infection studies.
In addition, the blood vessel cells of infected chips expressed higher levels of adhesion molecules, which allowed immune cells including B cells, T cells, and monocytes in the perfusion medium to attach to the blood vessel walls to help combat the infection. These results confirmed that the Alveolus Chip was mounting an immune response against H3N2 that recapitulated what happens in the lung of human patients infected with flu virus.
Focus on your breath
The team then carried out the same experiment without mechanical breathing motions. To their surprise, chips exposed to breathing motions had 50% less viral mRNA in their alveolar channels and a significant reduction in inflammatory cytokine levels compared to static chips. Genetic analysis revealed that themechanical strainhad activated molecular pathways related to immune defense and multiple antiviral genes, and these activations were reversed when the cyclical stretching was stopped.
"This was our most unexpected finding—that mechanical stresses alone can generate an innate immune response in the lung," said co-first author Longlong Si, Ph.D., a former Wyss Technology Development Fellow who is now a Professor at the Shenzhen Institute of Advanced Technology in China.
知道有时候肺部体验高雅r than 5% strain, such as in chronic obstructive pulmonary disorder (COPD) or when patients are put on mechanical ventilators, the scientists increased the strain to 10% to see what would happen. The higher strain caused an increase in innate immune response genes and processes, including several inflammatory cytokines.
"Because the higher strain level resulted in greater cytokine production, it might explain why patients with lung conditions like COPD suffer from chronic inflammation, and why patients who are put on high-volume ventilators sometimes experience ventilator-induced lung injury," Si explained.
From a chip to clinical trials
The scientists then went a step further, comparing the RNA molecules present in cells within strained vs. static Alveolus Chips to see if they could pinpoint how the breathing motions were generating an immune response. They identified a calcium-binding protein, called S100A7, that was not detected in static chips but highly expressed in strained chips, suggesting that its production was induced by mechanical stretching. They also found that increased expression of S100A7 upregulated many other genes involved in the innate immune response, including multiple inflammatory cytokines.
S100A7 is one of several related molecules known to bind to a protein on cells' membranes called the receptor for advanced glycation end products (RAGE). RAGE is more highly expressed in the lung than in any other organ in the human body, and has been implicated as a major inflammatory mediator in several lung diseases. The drug azeliragon is a known inhibitor of RAGE, so the scientists perfused azeliragon through the blood vessel channel of strained Alveolus Chips for 48 hours, then infected the chips with H3N2 virus. This pretreatment prevented the cytokine-storm-like response that they had observed in untreated chips.
Based on this promising result, the team then infected strained Alveolus Chips with H3N2 and administered azeliragon at its therapeutic dose two hours after infection. This approach significantly blocked the production of inflammatory cytokines—an effect that was further enhanced when they added the antiviral drug molnupiravir (which was recently approved for patients with COVID-19) to the treatment regimen.
These results caught the eye of Cantex Pharmaceuticals, which owns patent rights to azeliragon and was interested in using it to treat inflammatory diseases. Based in part on the Wyss team's work in Alveolus Chips, Cantex licensed azeliragon for the treatment of COVID-19 and other inflammatory lung diseases in early 2022. Given the drug's excellent safety record in previous Phase 3 clinical trials, the company has applied for FDA approval to start a Phase 2 trial in patients with COVID-19 patients, and plans to follow with additional Phase 2 trials for other diseases including COPD and steroid-resistant asthma.
"Thanks to the great work of the scientists at the Wyss Institute, we now have compelling evidence that azeliragon may have the potential to prevent severe COVID-19 illness in the form of a once-a-day pill. We're excited to have the opportunity to conduct clinical trials of azeliragon for this disease, seeking to bring this groundbreaking therapy to patients to prevent the life-threatening inflammation that is a major cause of hospitalization and death," said Stephen Marcus, M.D., CEO of Cantex.
While azeliragon is a promising anti-inflammatory drug, the scientists warn that more studies are needed to determine a safe and effective treatment regimen in humans. RAGE is a vital player in initiating beneficial inflammation against pathogens in the early stages of an infection, and inhibiting it too soon could prevent a patient from mounting a sufficient immune response.
Given the Alveolus Chip's many advantages over traditional preclinical models, the Wyss team is exploring the incorporation of additional cell types such as macrophages into the chips to increase their complexity and model more biological processes, such as adaptive immunity. They are also using their existing model to study the efficacy of new compounds, drugs, and biologics (such as mRNA therapeutics) against influenza, SARS-CoV-2, and other diseases.
"This important paper led to the discovery of RAGE inhibitors' promise for treating inflammatorylungdiseases, which was the basis for the recent license of azeliragon to Cantex and its movement toward human clinical trials for COVID-19. I am extremely proud of this team and how quickly this scientific finding was translated into commercialization that will hopefully lead to lifesaving treatment for patients. This is what the Wyss Institute is all about," said senior author Donald Ingber, M.D., Ph.D., who is the Wyss Institute's Founding Director as well as the Judah Folkman Professor of Vascular Biology at Harvard Medical School (HMS) and Boston Children's Hospital, and Hansjörg Wyss Professor of Bioinspired Engineering at the Harvard John A. Paulson School of Engineering and Applied Sciences.
More information:机械控制对先天免疫反应nst viral infection revealed in a human Lung Alveolus Chip,Nature Communications(2022).