XBB and BQ.1: what we know about these two omicron 'cousins'

In late October, theUK Health Security Agencyassigned variant designations to two new omicron "grandchildren": BQ.1 and XBB. This means they will be monitored by health authorities, but are not at this stage regarded as variants of concern.
If we think of theomicron variantas afamily tree, BA.2 (the dominant strain in the UK inspring of 2022) is the parent of BA.5 (the variantcurrently dominantin the UK) and the grandparent of BQ.1. In other words, BQ.1 is a sub-lineage of BA.5.
XBB is a hybrid of two omicron BA.2 lineages, BA.2.10.1 and BA.2.75. This makes XBB another grandchild of BA.2. XBB and BQ.1 are therefore cousins.
Ahybrid variantis created when two different sub-variants combine and swap parts of their genetic material. We've seen this happen with the coronavirus before, indicated by a variant name beginning with an "X" (like XD, XE and XF).
But what can we make of these variants? Are they cause for concern? Let's take a look first at how they're spreading.
In the UK, Europe and North America, the prevalence of BQ.1is rising quickly. Recent data from the UK'sOffice for National Statistics(ONS) estimated BQ.1 sub-lineages (including BQ.1 and the similar BQ.1.1) made up 16.7% of infections. In the US, BQ.1 and BQ.1.1 together make uparound 35% of infections.
XBB seems to be more prevalent in Asia. Whilethe ONS datasuggests it makes up only 0.7% of infections in the UK, in Singaporesome 58%of recently sequenced cases are XBB. But whereas sequences of XBB areincreasing globally, XBB cases in Singapore now appear to bestarting to fall.
Scientists are closely watching several regions where both variants are circulating to see which has the competitive edge.
What are the differences between BQ.1 and XBB?
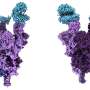
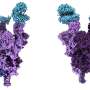
Omicron variants are successful due in part to several shared mutations in the receptor-binding domain of the spike protein (a protein on the surface of the virus which allows it to attach to our cells).
A key difference between BQ.1 and XBB is the number and location of mutations in the receptor-binding domain. This part of the protein is required for the virus to infect our cells, and is also the target of antibodies which are an important part of the immune response.
A recent preprint (a study yet to be peer-reviewed) suggests that mutations withinthe receptor-binding domaincan help XBB escape neutralising antibodies generated by COVID vaccines or infection with earlier omicron offshoots, including the parent strains BA.2 and BA.5. This preprint regards XBB as one of the most antibody-evasive coronavirus strains we've seen.
Cornelius Roemer, a computational biologist in Switzerland,has suggestedthat a number of these specific changes in the receptor-binding domain can increase the virus's growth efficiency, or "fitness". The idea is that more changes allow it to attach tohuman cellsmore strongly, giving it a better chance of infecting that cell.
According to Roemer's model, BQ.1 is a level-5 variant, with five mutations from a list of 21 key mutations which increase SARS-CoV-2's ability to strongly attach to human cells. XBB is currently the only variant at level 7, with seven of the 21 key mutations.
At the moment this is just an observation and hasn't been formally published or peer reviewed. However,other scientistshave also noted XBB's apparent growth advantage over other variants.
But it's not just mutations in the receptor-binding domain that could give XBB an edge. XBB has mutations in another part of the spike protein called the N-terminal domain as well.
Theimmune systemalso targets the N-terminal domain with antibodies, and people who have recovered fromBA.2andBA.5infections mount especially strong immune responses to this part of the spike protein. Preliminary evidence suggests XBB is very effective atevading these antibodiestoo. This means that previous infection with BA.2 or BA.5 might not protect you from XBB.
Should we be worried about XBB?
By these metrics, XBB is potentially more immune evasive than BQ.1 and its parent BA.5, and might have a growth advantage which could increase virus spread.
The good news is that based on Singaporean data, XBB has been estimated to have a30% lower risk of hospitalisationcompared with BA.5. But we don't yet have data to support this from other countries, so this might change as XBB becomes more widespread.
有可能我们可能面临一波infections in the UK—a wave of BQ.1 infections from Europe and the US, before a second wave of XBB from Asia. And we don't know if BQ.1 will offer any protection against XBB. Only time will tell whether XBB will outperform BA.5 or BQ.1, or if anothervariantis waiting in the wings.
This article is republished fromThe Conversationunder a Creative Commons license. Read theoriginal article.![]()