This article has been reviewed according to Science X'seditorial processandpolicies.Editorshave highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Cell mapping and 'mini placentas' give new insights into human pregnancy
剑桥大学的研究人员Wellcome Sanger Institute, the Friedrich Miescher Institute for Biomedical Research (FMI), Switzerland, EMBL's European Bioinformatics Institute (EMBL-EBI), and collaborators, have created an in-depth picture of how the placenta develops and communicates with the uterus.
The study, published today in the journalNature, is part of the Human Cell Atlas initiative to map every cell type in thehuman body. It informs and enables the development of experimental models of the human placenta.
"For the first time, we have been able to draw the full picture of how the placenta develops and describe in detail the cells involved in each of the crucial steps. This new level of insight can help us improve laboratory models to continue investigating pregnancy disorders, which cause illness and death worldwide," said Anna Arutyunyan, co-first author at the University of Cambridge and Wellcome Sanger Institute.
The placenta is a temporary organ built by the fetus that facilitates vital functions such as fetal nutrition, oxygen and gas exchange, and protects against infections.The formation and embedding of the placenta into the uterus, known as placentation, is crucial for a successful pregnancy.
Understanding normal and disordered placentation at amolecular levelcan help answer questions about poorly understood disorders including miscarriage, stillbirth, and pre-eclampsia. In the UK, mild pre-eclampsia affects up to six percent of pregnancies. Severe cases are rarer, developing in about one to two percent of pregnancies.
Many of the processes in pregnancy are not fully understood, despite pregnancy disorders causing illness and death worldwide. This is partly due to the process of placentation being difficult to study in humans, and while animal studies are useful, they have limitations due to physiological differences.
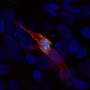
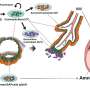
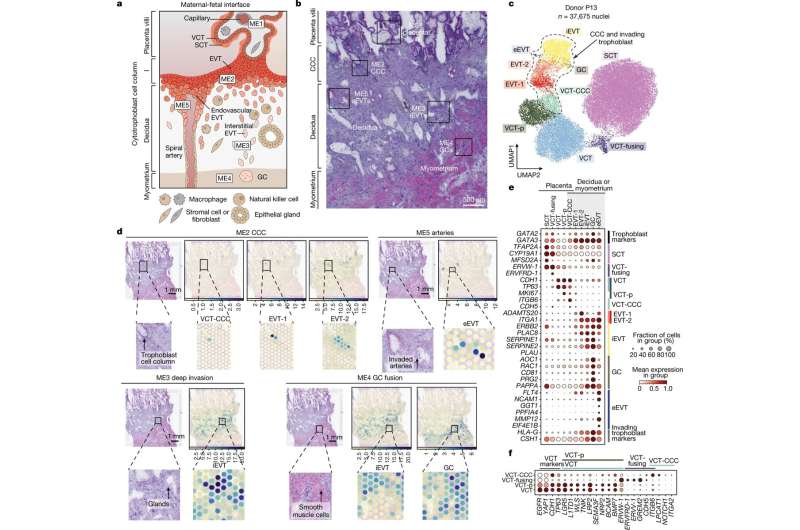
During its development, the placenta forms tree-like structures that attach to the uterus, and the outer layer of cells, called trophoblast, migrate through the uterine wall, transforming the maternal blood vessels to establish a supply line for oxygen and nutrients.
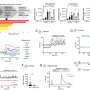
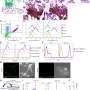
In the new study, scientists built on previous work investigating the early stages of pregnancy, to capture the process of placental development in unprecedented detail. Cutting-edge genomic techniques allowed them to see all of thecell typesinvolved and how trophoblast cells communicate with the maternal uterine environment around them.
The team uncovered the full trajectory of trophoblast development, suggesting what could go wrong in disease and describing the involvement of multiple populations of cells, such as maternal immune and vascular cells.
"This research is unique as it was possible to use rare historical samples that encompassed all the stages of placentation occurring deep inside the uterus. We are glad to have created this open-access cell atlas to ensure that thescientific communitycan use our research to inform future studies," said Professor Ashley Moffett, co-senior author at the University of Cambridge's Department of Pathology.
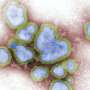
They also compared these results to placental trophoblast organoids, sometimes called "mini-placentas," that are grown in the lab. They found that most of the cells identified in the tissue samples can be seen in these organoid models. Some later populations of trophoblast are not seen and are likely to form in the uterus only after receiving signals from maternal cells.
The team focussed on the role of one understudied population of maternal immune cells known as macrophages. They also discovered that other maternal uterine cells release communication signals that regulate placental growth.
The insights from this research can start to piece together the unknowns about this stage of pregnancy. The new understanding will help in the development of effective lab models to study placental development and facilitate new ways to diagnose, prevent, and treatpregnancydisorders.
More information:Anna Arutyunyan et al, Spatial multiomics map of trophoblast development in early pregnancy,Nature(2023).DOI: 10.1038/s41586-023-05869-0